|
Qpack, IQ och OQ validering,
alltid i samband med installation, men också
som en del av våra serviceavtal.
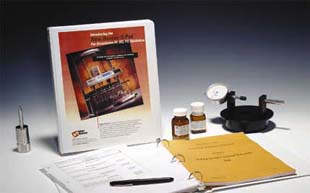
Q-Pak™
Validation Guidelines
Q-Paks provide functional
validation guides to ensure USP/FDA compliance and
system IQ/OQ/PQ. These unique guidelines direct the
user through instrument qualification with a comprehensive
logbook documenting all key elements of functional
validation. Combined with our 21CFR11 firmware, SOPs
and easy-to-use calibration tools, the Q-Pak is an
ideal means to satisfy full regulatory compliance.
|
